

The cookie is used to store the user consent for the cookies in the category "Performance". This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other.
The cookies is used to store the user consent for the cookies in the category "Necessary". The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". The cookie is used to store the user consent for the cookies in the category "Analytics". These cookies ensure basic functionalities and security features of the website, anonymously. Necessary cookies are absolutely essential for the website to function properly. As a result of the geometry of the sp hybrid orbitals, the two carbon atoms of the triple bond and the two atoms directly attached are collinear. What bonds are in a triple bond?Ī triple bond in an alkyne consists of one sigma bond and two pi bonds. What is meant by a single bond?ĭefinition of single bond : a chemical bond in which one pair of electrons is shared by two atoms in a molecule especially when the atoms can share more than one pair of electrons - compare double bond, triple bond. We can see the formation of a double bond inLewis dot structure of Carbon dioxide.
#SIGMA BOND EXAMPLE FULL#
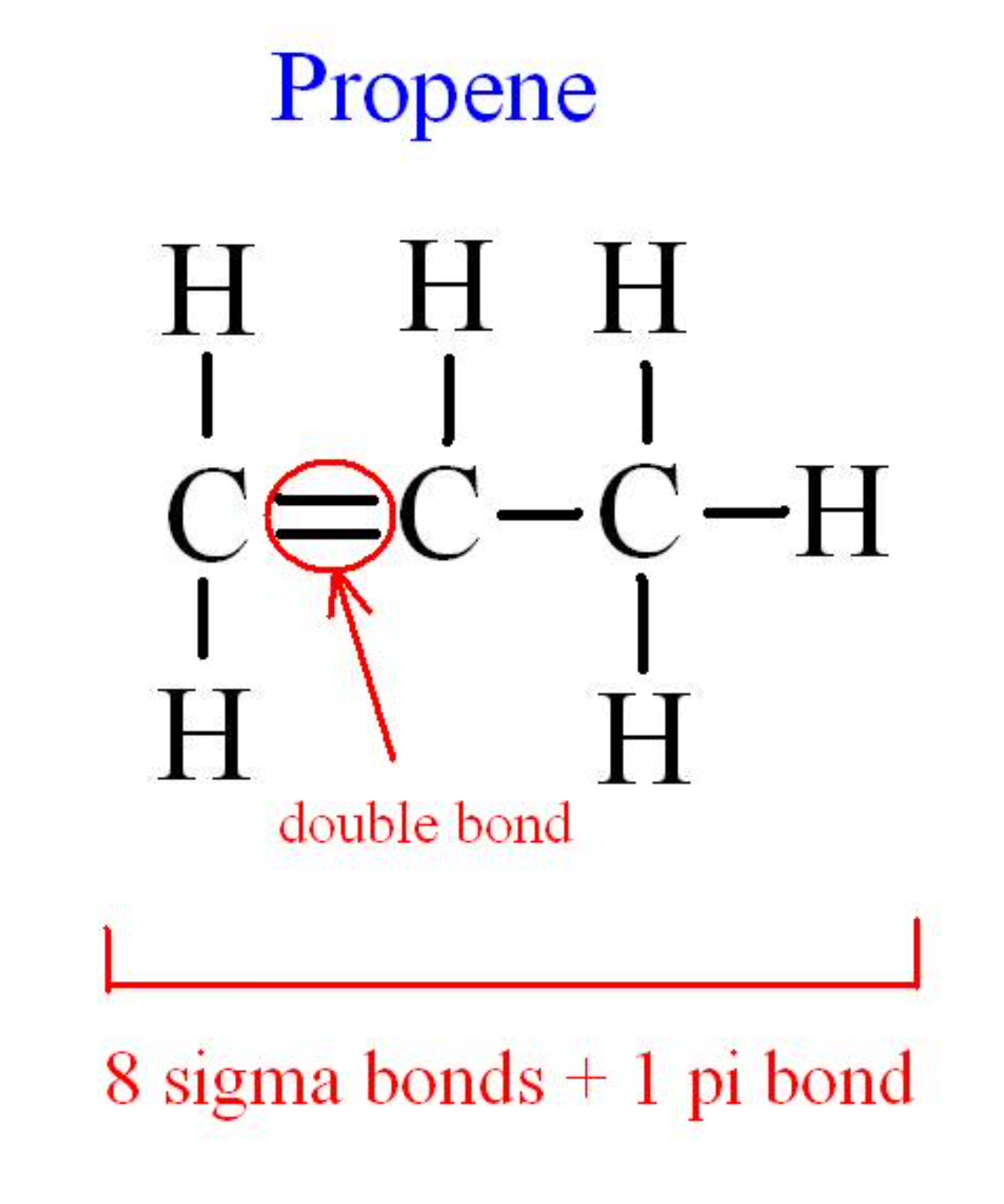
Double bond occurs when 2 pairs of electrons are shared between the atoms to attain a full outer shell. Pi bonds can form in double and triple bonds but do not form in single bonds in most cases. How many σ and π bonds are in each compound?įrequently Asked Questions about Sigma and Pi Bonds Every single bond is 1 sigma bond, every double bond has 1 sigma and 1 pi bond, and every triple bond has 1 sigma bond and 2 pi bonds. 90% of all bonds described some time or another are somehow involving carbon, nitrogen or oxygen. It cannot be formed by s-orbitals it needs at least p-orbitals to be created. Why are pi bonds only p orbitals?Ī π bond has a plane of symmetry along the bond axis.

This is because, bond strength is directly proportional to the directional nature of orbitals in overlapping. Yes, generally p-p sigma bond is stronger than s-s sigma bond. Thus, the total number of sigma bonds is 12. In benzene, there are 6 (C-C) sigma bonds and 6 (C-H) sigma bond. The number of sigma bonds present in triple bonds is only one. Triple bond is defined as the chemical bond between two atoms that have six bonding electrons. The number of sigma bonds present is one and the number of pi bonds present in a double bond is one. It forms four bonding electrons between the atoms. Pi bonds are chemical bonds that are covalent in nature and involve the lateral overlapping of two lobes of an atomic orbital with two lobes of another atomic orbital that belongs to a different atom. Hence, pi bonds are weaker than sigma bonds. In pi bonds, overlapping takes place at the side of the two lobes of p – orbitals so the extent of overlapping is less than the sigma bond. Pi bonds are formed by sideways overlapping of two parallelly oriented pi orbitals of adjacent atoms.
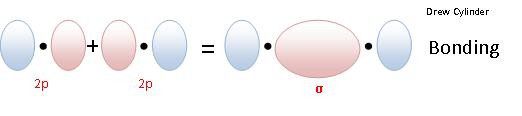
This is different from a pi bond where they touch sideways. In a sigma bond, the atomic orbitals from two atoms which are nearby go one on top of each other. It is the strongest one of the covalent type. What is a sigma bond in simple terms?Ī sigma bond (σ bond) is a type of chemical bond. The bonds between the sp3 orbitals of hybridized carbon and the s orbitals of hydrogen in methane are also example of sigma bonds. The bond between two hydrogen atoms is an example of sigma bonding. Pi bond, in chemistry, a cohesive interaction between two atoms and a pair of electrons that occupy an orbital located in two regions roughly parallel to the line determined by the two atoms. 24 What bonds are in a triple bond? What is pi bond in chemistry?


 0 kommentar(er)
0 kommentar(er)
